Molecular Cheese Sauce

Can be cooked on a gas hob, or open fire embers
Make processed cheese, just like the Big Cheese factories do. It’s still cheese, just in a more fun form
4 serves
Ingredients
- 100 g grated or crumbled cheese (~1 cup)
- 60 ml fluid (milk, water, or beer) (~1¼ cup, subtract fluid Sodium Citrate is dissolved in)
- 4 g Sodium Citrate (~½ tsp)
Method
Add the cheese, half the fluid, and the sodium citrate to a heatproof bowl. Use either a double boiler or microwave oven to gently heat until the cheese fully melts
Gently fork or whisk until it forms a smooth creamy sauce. This may take two to five minutes, and may require extra heating cycles
Add extra fluid until the sauce has the consistency you want, going past the amount specified will cause the sauce to split
Any cheese will work, but typically it works best with basic cheese like Cheddar, Colby, Edam, and Mozzarella. Mixes of different cheeses can of course be used; Mozzarella and blue anyone?
Sodium Citrate
This is the emulsifier; it stops the cheese oils from separating when heated. Classic white sauce uses flour as the emulsifier, but flour does not work well with cheese, and changes the taste
While you can buy Sodium Citrate from restaurant food suppliers, it’s not common in grocery shops, so the easy way is to make it
The simplest method is to use the juice of one medium lemon (44 ml) in a heatproof mixing jug. This contains about 3 g of Citric Acid. Mix in 4 g of Baking Soda (½ tsp). This will produce about 4 g of Sodium Citrate in a fluid form, with a nice lemon fragrance. It tastes a bit weird, but you will not notice this when it has emulsified the cheese oils
The other method is to dissolve 3 g of Citric Acid (½ tsp). You can buy Citric Acid in the spice section of the supermarket. Mix with 4 g of Baking Soda (½ tsp) in 10 ml of hot water
Caution: both methods will foam up. Briefly heat the mix until the foaming stops
Fun Stuff
Make this cheese sauce with the minimum liquid, usually requiring a little more heat. Pour the mix onto non-stick baking paper and chill it to make your own processed cheese slices. Cut out with cookie cutters for even more fun
The cold setting firmness can be improved by adding 0.25 g gelatin or carrageenan
Chemistry Time
Making your own Sodium Citrate is simple chemistry. By molecular weight we mix three parts of Sodium Bicarbonate, with one part of Citric Acid
The components
Sodium Bicarbonate is normally called Baking Soda, and is commonly used in cooking. Baking Powder consists of about 50% Baking Soda
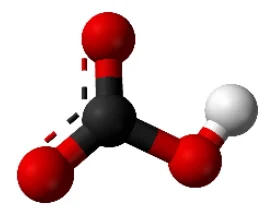
The chemical formula is NaHCO3. This means one atom of Sodium (Na), one atom of Hydrogen (H), one atom of Carbon (C), and three atoms of Oxygen (O) combined. Sodium is a soft silvery metal, what is violently reactive in it pure form. You normally consume about 5 g per day of it in the form of common table salt, chemically named Sodium Chloride (NaCl)
Citric Acid is the main ingredient in Citrus fruits. Lemons have the most, Limes not far behind. Citric Acid is the sour taste you get when eating or drinking a lemon
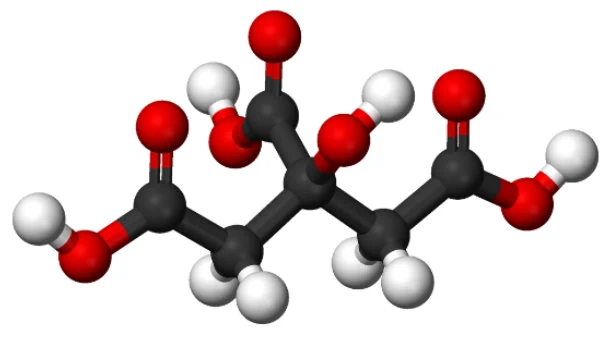
Its chemical formula is C6H8O7. This means six atoms of Carbon (C), eight atoms of Hydrogen (H), and seven atoms of Oxygen (O). Carbon, Hydrogen, and Oxygen are the main components for the human body. H2O is the water you drink, and sugar or carbohydrate is a form of C6H12O6 or C8H10O5
The Formula
When we mix three parts of Sodium Bicarbonate, with one part of Citric Acid, we get one part of Sodium Citrate (Na3C6H5O7), three parts of water (H2O), and three parts of Carbon Dioxide (CO2) gas, the same gas in your favourite fizzy drinks
We can check the balance of the chemical reaction with this table
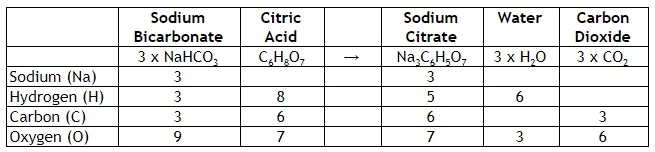
The left and right hand sides are equal for each component, so it’s a valid reaction. E.g. for Oxygen; Sodium Bicarbonate x 3 + Citric Acid has 16 atoms. Sodium Citrate + Water x 3 + Carbon Dioxide x 3 = 16 atoms. This is balanced, and matter is not created or destroyed, just re-mixed
How much to use?
Four grams of Sodium Bicarbonate (~½ tsp) has 602,214,129,000,000,000,000,000 molecules, or about 50,585,986,836,000,000,000,000,000 atoms. These numbers are a bit large to work with, so in chemistry we work in moles, which is a simple representation of these large numbers by using the weight divided by the Molar Mass (total atomic weight of the chemical components)
What is the atomic weight? It’s the average count the atoms internal parts (protons, neutrons, and electrons)
- Sodium (Na) - 23
- Hydrogen (H) - 1
- Carbon (C) - 12
- Oxygen (O) - 16
We then multiply that out by the molecules, we get
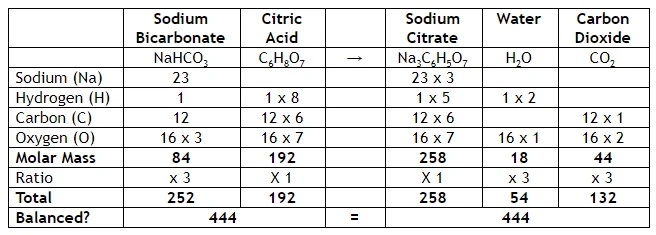
We balance by adding up the left and right hand side, and check if they are equal
The ratio in the formula is 3 parts Sodium Bicarbonate mixed with 1 part of Citric Acid to make 1 part Sodium Citrate, and some other stuff
For this recipe we have 4 g of Sodium Bicarbonate, and 3 g of Citric Acid. Those amounts were calculated using moles
To work out moles, we take the weight and divide this by the Molar Mass. This is 4 (g) / 84 (atomic weight) / 3 (formula ratio) = 0.0159 moles of Sodium Bicarbonate, and 3 (g) / 192 (atomic weight) / 1 (formula ratio) = 0.0156 moles of Citric Acid
The moles match (close enough), so the reaction is full and complete, and there is no left over un-reacted chemical
This is much easier than counting out 602,214,129,000,000,000,000,000 Sodium Bicarbonate molecules!
Published